- ホーム
- 【English】Human Stem Cell Culture Supernatant (Exosome-Enriched) Therapy
【English】Human Stem Cell Culture Supernatant (Exosome-Enriched) Therapy
What is Human Stem Cell Culture Supernatant?
Human stem cell culture supernatant is a purified fluid obtained during the cultivation and proliferation of human stem cells. Although it does not contain actual stem cells, this supernatant is rich in bioactive components such as a wide variety of growth factors, cytokines (including immune modulatory, anti-inflammatory, and neuroregenerative factors), and exosomes. These components have been shown to contribute to the repair of damaged or aged cells, suppression of inflammation, and regeneration of blood vessels and nerves. As such, it is expected to serve as a complementary or alternative approach to stem cell therapy.
At our clinic, we provide safe and effective treatments using stem cell culture supernatant from highly reliable and carefully selected manufacturers. All preparations are thoroughly evaluated in terms of ingredient standards, concentration, and safety testing before use in clinical applications.
※Cytokines are signaling molecules that regulate cellular growth and immune responses by transmitting information between cells. Exosomes are small vesicles secreted outside of cells that contain growth factors and other bioactive substances. They play a role in cell-to-cell communication and tissue regeneration. Both are gaining attention in the field of regenerative medicine due to their potential to deliver therapeutic effects similar to stem cell treatments.
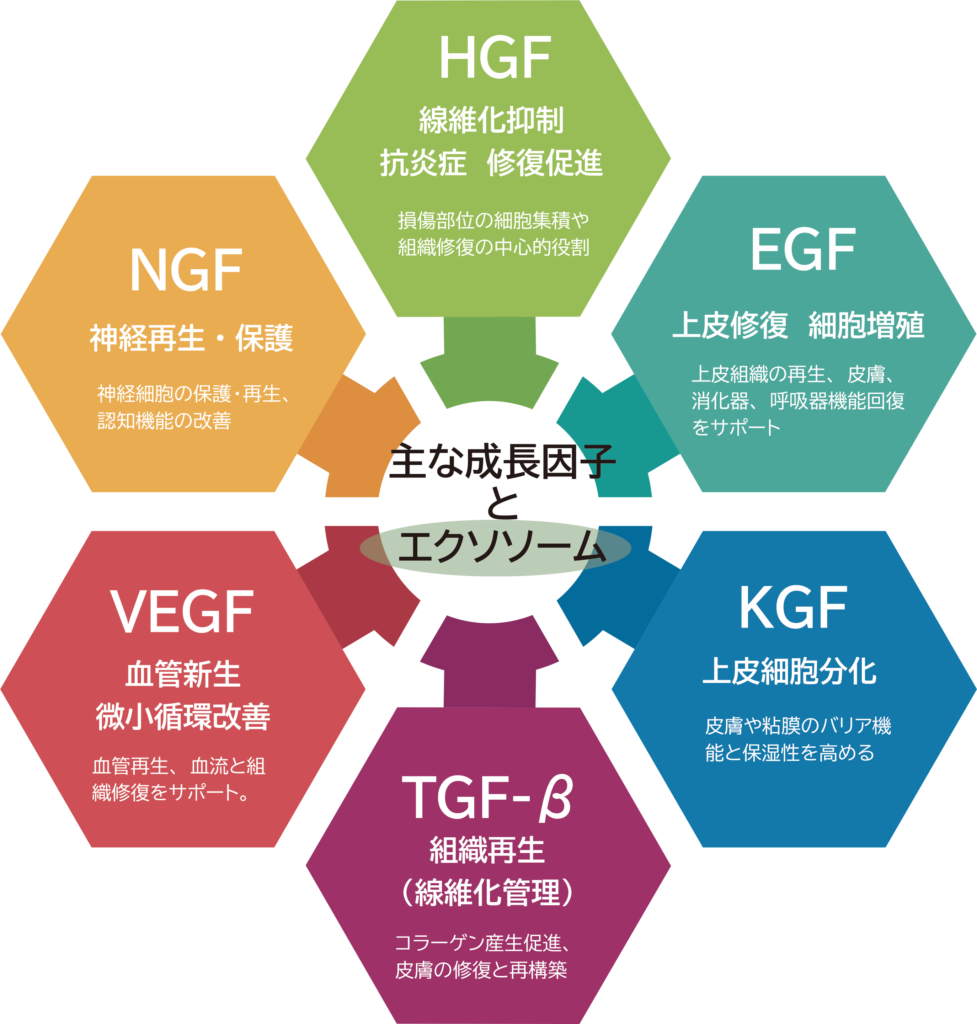
Key Growth Factors Contained in Stem Cell Culture Supernatant
- HGF (Hepatocyte Growth Factor) Anti-fibrotic, anti-inflammatory, and tissue repair promoting:
- NGF (Nerve Growth Factor) Neural regeneration and protection:
- VEGF (Vascular Endothelial Growth Factor) Angiogenesis and microcirculation improvement:
- EGF (Epidermal Growth Factor) pithelial repair and cell proliferation:
- KGF / FGF-7 (Keratinocyte Growth Factor) nduction of epithelial cell differentiation:
- TGF-α (Transforming Growth Factor Alpha) Tissue regeneration and fibrosis modulation:
- Exosomes These are extracellular vesicles containing growth factors and other signaling molecules. They facilitate intercellular communication and contribute to tissue regeneration.
HGF facilitates cell migration and survival, playing a central role in the accumulation of cells at injury sites and promoting tissue repair. It also exhibits anti-fibrotic properties and is involved in the regeneration of organs such as the liver, lungs, and kidneys following damage.
NGF suppresses cell death during injury and supports the growth, maintenance, and regeneration of nerve cells through axonal elongation and neurite outgrowth. It has also been reported to be effective in improving cognitive function.
VEGF promotes the proliferation of endothelial cells and the formation of new blood vessels, enhancing blood flow to tissues. It supports wound healing by improving oxygen and nutrient delivery and reducing ischemia.
EGF stimulates the proliferation and migration of epithelial progenitor cells, aiding in the restoration of functions in epithelial tissues such as the skin, gastrointestinal tract, and respiratory tract.
This factor selectively acts on keratinocytes, promoting their proliferation and differentiation. It contributes to the barrier function, moisture retention, and structural stability of the skin and mucous membranes.
TGF-α stimulates fibroblasts in the skin to produce essential structural components like collagen and elastin. It supports tissue reconstruction and structural stability during the repair process.
Our Commitment to Quality and Safety
- Carefully Selected Human Stem Cell Culture Supernatant (Exosome-Enriched)
All of the supernatant we use is derived from human sources. For adipose tissue– and dental pulp–derived supernatant, the donors are healthy Japanese individuals. For umbilical cord–derived supernatant, the donors are healthy Korean individuals. - Commitment to Safety
The supernatant solutions we select are manufactured either in university research laboratories within Japan or in Class 100 cleanroom cell processing centers (CPCs), all under strict quality control. They undergo advanced purification to remove impurities and are rigorously tested for viruses and sterility to ensure the highest level of safety. - Use of Adipose-Derived Supernatant Rich in HGF
We use adipose-derived human stem cell culture supernatant (exosome-enriched), produced using a proprietary concentration technique that yields the highest levels of HGF (hepatocyte growth factor). HGF is one of the most important cytokines due to its multifaceted effects, including tissue repair and regeneration (especially in the liver, kidneys, and lungs), anti-inflammatory action, anti-fibrotic effects (through TGF-β suppression), and neuroprotective properties. Whether administered alone or in combination with other factors, HGF-based therapy has drawn considerable attention. Thanks to its abundant HGF content, this supernatant is expected to promote organ repair, vascular and tissue regeneration, as well as improvement in skin and hair quality.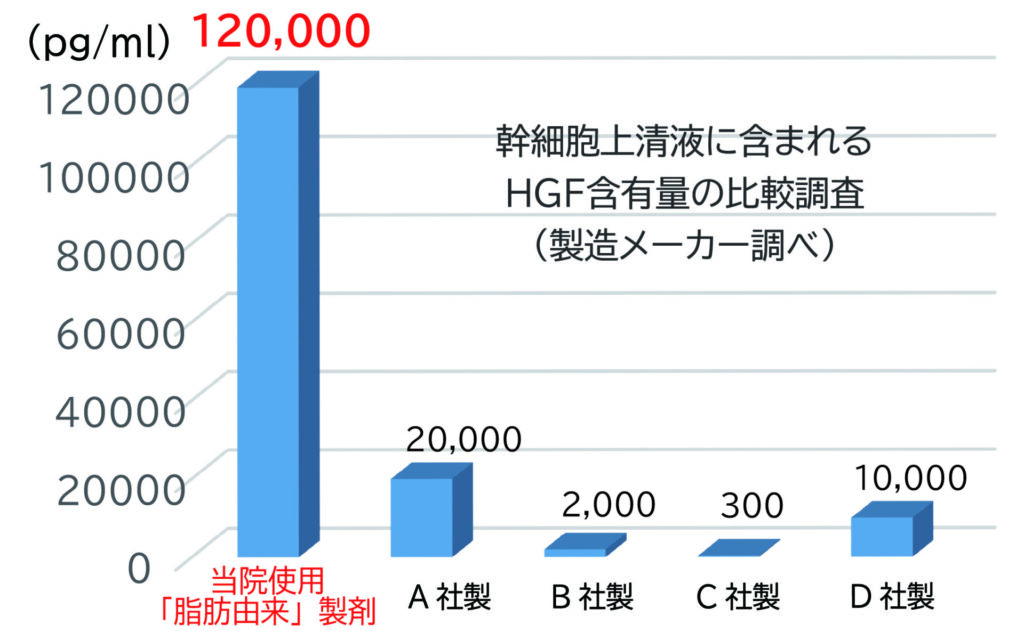
We also implement rigorous purification processes to remove not only impurities, viruses, and bacteria, but also unnecessary DNA fragments. All stem cell donors are healthy Japanese women, and production is centrally managed within university-based laboratories in Japan. - Combined Intravenous and Intranasal Therapy for Cognitive Function
For cognitive enhancement, a combination of intravenous administration of adipose-derived supernatant and intranasal delivery has been shown to be effective. In a clinical trial using intranasal administration of stem cell–derived conditioned medium, cognitive scale scores improved in 18 patients with mild to moderate Alzheimer’s disease.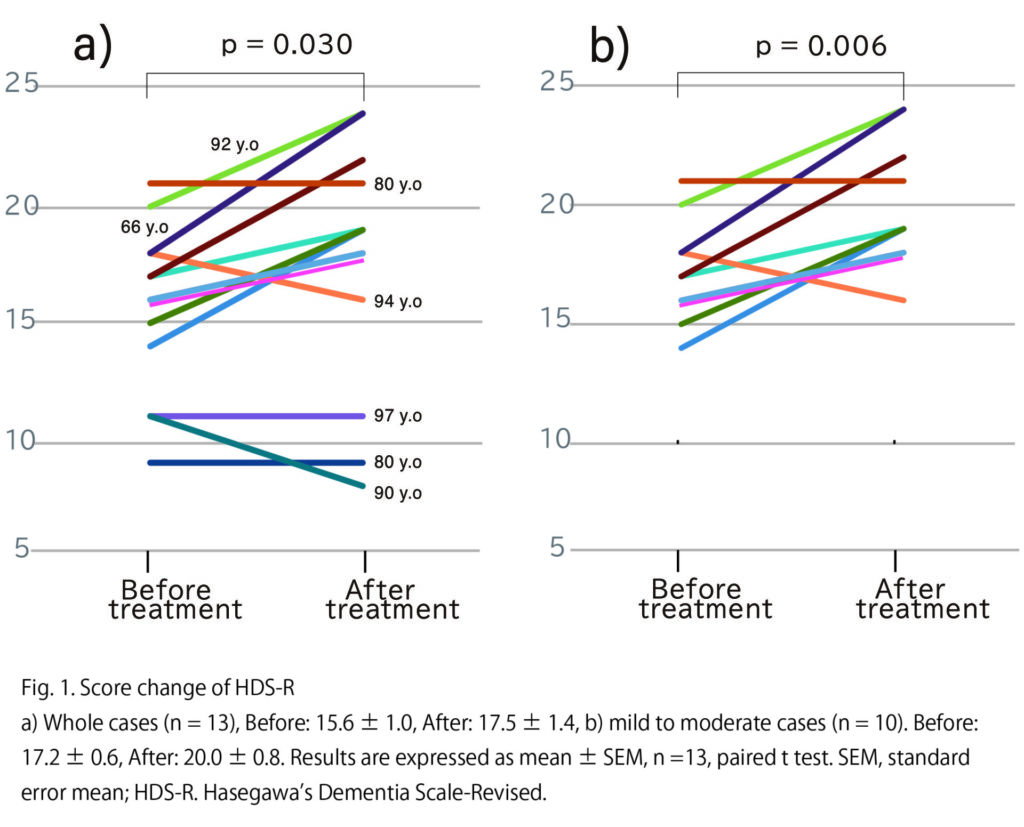
※Reference: “Safety and clinical efficacy on intranasal administration of mesenchymal stem cell-derived secretome in patients with Alzheimer’s disease and its future prospect.” Morita Y, Izawa H, Ohga H, Sugie H, Yamamoto T, Hirano A, Nakamura M, Isozaki S, Seino K, Yonei Y. Glycative Stress Research, 2024; 11(3):103–110. (Adapted from https://www.toukastress.jp/webj/article/405)
- Treatment Options Tailored to Your Needs
In addition to adipose-derived supernatant, known for its broad-spectrum regenerative potential, we also offer options derived from dental pulp and umbilical cord to suit various treatment objectives. - Application in Aesthetic Medicine
As an optional add-on, our stem cell culture supernatant can be used in aesthetic treatments via electroporation using the Mesonα+ (Mesona Plus) system. This includes scalp application for hair restoration (HART-F) and facial treatments for skin rejuvenation.
Indications for Therapy
- Frequent fatigue or a general sense of aging (both physically and mentally)
- Feeling unwell or lacking vitality
- Desire to maintain a youthful appearance and function
- Noticeable thinning of hair or visible scalp
- Excessive hair shedding
- Presence of dullness, wrinkles, spots, or melasma
- Loss of skin elasticity, firmness, and glow
A More In-Depth Explanation>What Are Growth Factors and Cytokines?
Details
Growth factors and cytokines are signaling molecules that regulate cellular activity by modulating gene transcription within the cell nucleus, thereby initiating a wide range of physiological responses throughout the body. Growth factors, in particular, play essential roles in numerous biological processes in adults, including cell proliferation, differentiation, and apoptosis, as well as immune modulation, hematopoiesis, angiogenesis, metabolism, wound healing, and maintenance of tissue homeostasis. These signals are typically released in response to stimuli received by the secreting cells and act upon specific target cells to exert their physiological effects.
A More In-Depth Explanation>What Are Exosomes?
Details
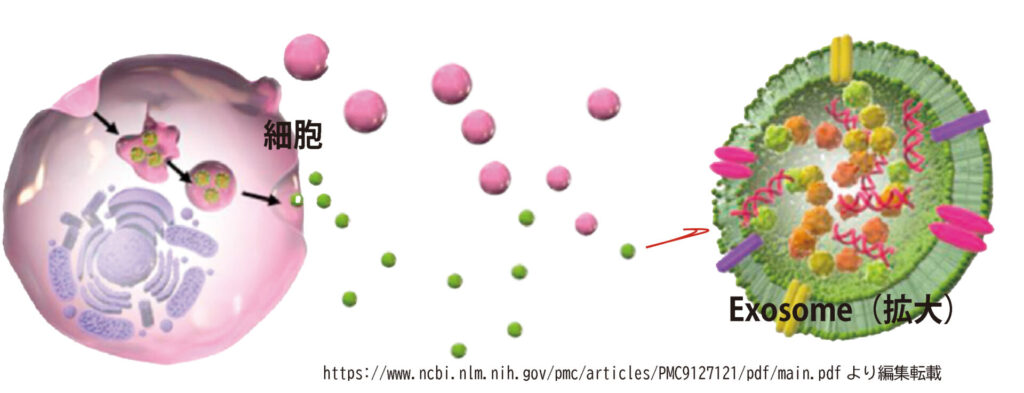
Exosomes are a type of extracellular vesicle (EV) secreted by cells, and they contain biologically active substances such as growth factors and signaling molecules essential for tissue regeneration and intercellular communication.
Exosomes play a vital role in mediating cell-to-cell signaling and have gained increasing attention for their therapeutic potential in various disease states. In the field of regenerative medicine, exosomes derived from mesenchymal stem cells (MSCs) are of particular interest. These MSC-derived exosomes exhibit a broad spectrum of therapeutic effects, including anti-inflammatory and anti-fibrotic actions, promotion of angiogenesis, and acceleration of tissue repair. As such, they are regarded as promising tools for “cell-free therapy”—an emerging concept in regenerative treatment.
For example, in myocardial infarction models, MSC exosomes have been shown to suppress cardiomyocyte apoptosis, promote new blood vessel formation, and reduce inflammation. In other preclinical models such as chronic kidney disease, pulmonary fibrosis, and arthritis, MSC-derived exosomes have also demonstrated protective and regenerative effects on tissues.
These beneficial actions are believed to be mediated by the microRNAs (miRNAs) and proteins contained within the exosomes, which modulate gene expression in recipient cells. Given these mechanisms, exosomes are increasingly anticipated to serve as a complement to or even substitute for conventional stem cell therapies.
Late-Phase Clinical Trials Involving Exosome-Based Therapies (Adipose-Derived)
Details
Several human clinical trials have investigated the use of exosomes and related extracellular vesicles in the treatment of various inflammatory and degenerative conditions. Although the field is advancing rapidly, it is important to recognize that scientific evidence remains under development. Many medical institutions are already offering exosome-based treatments, but at this stage, clinical application should proceed with appropriate caution and rigorous oversight.Examples of Phase 2/3 Trials:
• Efficacy and Safety of EXOSOME-MSC Therapy to Reduce Hyper-inflammation in Moderate COVID-19 Patients
Investigating the potential of exosome-based MSC therapy to reduce excessive inflammation in moderate COVID-19 cases.
• Effect of Microvesicles and Exosomes Therapy on β-cell Mass in Type I Diabetes Mellitus (T1DM)
Evaluating whether therapy with microvesicles and exosomes can improve or preserve pancreatic β-cell mass in T1DM patients.
• The Effect of Stem Cells and Stem Cell Exosomes on Visual Functions in Patients With Retinitis Pigmentosa
Studying the impact of stem cells and their exosomes on visual function in patients with degenerative retinal disease.
• Use of Autologous Plasma Rich in Platelets and Extracellular Vesicles in the Surgical Treatment of Chronic Middle Ear Infections
Assessing the clinical outcomes of applying platelet- and EV-rich autologous plasma in surgical management of chronic otitis media.
Example of a Phase 3 Trial:
Extracellular Vesicle Treatment for Acute Respiratory Distress Syndrome (ARDS) (EXTINGUISH ARDS)
A large-scale trial evaluating the use of extracellular vesicles in patients with ARDS, a life-threatening pulmonary condition.
Product Specifications and Safety Testing Results
[Adipose-Derived Human Stem Cell Conditioned Media Extract]
Overview
Details
Product Name:
Human Adipose Stem Cell Conditioned Media Extract (Freeze-dried)
(Purified extract of human adipose-derived stem cell culture supernatant)
Classification: Research reagent
Origin: This product is a freeze-dried formulation of a purified and concentrated solution derived from the culture medium of human adipose-derived stem cells.
Country of Manufacture: Japan
| Manufacturing Details | Processing Facility: Cell Processing Center (CPC) attached to a university hospital (GMP-compliant) |
|---|---|
| Form | Undiluted solution |
| Country of Manufacture | Japan |
| Donor | Japanese |
| Donor Screening (Infectious Disease) | 7 items |
| Donor Blood Testing Frequency | Once |
| Viral Testing (Final Product) | Performed |
| Mycoplasma Testing | Negative |
| Sterility Testing | Negative |
| Endotoxin Testing | Below 0.015 EU/mL |
| Product Management | By lot |
| Exosome Quantification | – |
Analytical Parameters
Details
Appearance:
White powder; colorless to pale yellow, odorless solution upon reconstitution
TBU (Total Bioactive Units): ≥120 TBU (ELISA method)
NH₃ (Ammonia): <10 µg/dL (upon dissolution in 2 mL, indicator method)
pH: 7.4 ± 0.3 (upon dissolution in 2 mL, glass electrode method)
Heavy Metals: Not detected (sodium sulfide colorimetric method)
Arsenic: Not detected (atomic absorption spectrophotometry)
Storage Conditions:
Store refrigerated (2–6°C) in a sealed container away from direct sunlight
Shelf Life:3 years from the date of manufacture(Once opened, use promptly to avoid contamination.)
Allergens: None contained
GMO Status: Not applicable
Animal-derived Components: Not contained
Safety Testing Results
Details
1.Microbiological Testing
・Sterility Test
(SCDLP and GPLP agar plate method): Negative — Compliant
・Mycoplasma Test
(Nucleic Acid Testing, NAT): Negative — Compliant
・Endotoxin Test
(Gel clot method): <0.015 EU/mL — Compliant
2.Primary Skin Irritation Test(LabCyte EPI-MODEL24 SIT test using a 3D cultured human skin model)
Summary:
The test substance showed a cell viability of 101.1 ± 1.1%, well above the 50% threshold, indicating no irritation.
Therefore, a 10% solution of the freeze-dried extract was classified as “Not Classified” (non-irritant) for skin irritation.
3.Acute Oral Toxicity Test (Mouse Study)
Summary:
A single oral dose of the product dissolved in distilled water (2000 mg/kg) was administered to groups of 5 male and 5 female mice.
The animals were observed for 14 days post-administration, with necropsy performed on Day 15.
No signs of toxicity were observed at the maximum dose tested.
Viral Screening
Details
1)Virus Test Method Result
・Hepatitis B Virus (HBV) HBs Antigen, HBc Antibody (CLIA) Negative — Compliant
・Hepatitis C Virus (HCV) HCV Antibody II (CLIA) Negative — Compliant
・Human Immunodeficiency Virus (HIV) HIV Antigen/Antibody (CLIA) Negative — Compliant
・Human T-cell Leukemia Virus (HTLV-1) HTLV-1 Antibody (CLEIA) Negative — Compliant
・Human Parvovirus B19 (PVB-19) PVB-19 DNA (PCR) Negative — Compliant
2)Human Parvovirus B19 (PVB-19) PVB-19 DNA (PCR) Negative — Compliant
HBV Antigen (HBsAg) CLIA Negative — Compliant (<0.005 IU/mL)
HBV Antibody (HBsAb) CLIA Negative — Compliant (<0.1 COI)
HCV Antibody II CLIA Negative — Compliant (<0.1 COI)
HIV Antigen CLIA Negative — Compliant
HIV Antibody CLIA Negative — Compliant
HTLV-1 Antibody CLIA Negative — Compliant
PVB-19 DNA PCR Negative — Compliant
Source: Manufacturer’s Technical Report
Treatment Process
- Consultation The physician will assess your symptoms and concerns during the consultation and determine the appropriate method and dosage for administration.
- Administration (IV Drip / Injection) Injections typically take around 5 minutes, while IV drips require approximately 30 to 60 minutes to complete.
Frequency & Duration of Treatment
IV drips are generally recommended once every 1 to 4 weeks. While some individuals may notice effects after a single session, many experience progressive benefits with repeated treatments. Localized injections (e.g., intra-articular) may be used to manage chronic pain by delivering the conditioned medium directly to the affected area.
※For optional delivery methods such as HART-F and Mesona Plus, please refer to the respective treatment pages for detailed process information.Side Effects & Risks:
Mild pain or bruising may occur at the injection or IV site.
※For optional delivery methods such as HART-F and Mesona Plus, please refer to the respective treatment pages for detailed process information.Treatment Fees
Human Stem Cell Culture Supernatant (Exosome-Enriched) Therapy
【Adipose-Derived Supernatant】Rich in various growth factors such as HGF and abundant in exosomes, this formulation offers wide-ranging regenerative effects, including organ repair, vascular and tissue regeneration, and improvements in skin and hair quality. For cognitive function support, combining intravenous infusion and intranasal administration—both derived from adipose tissue—is considered highly effective.
<Administration Methods>
IV Drip Infusion: For systemic anti-aging and overall health maintenance
Local Injection: For targeted relief of chronic pain (e.g., intra-articular)
| Treatment | Expected Effects | Price (incl. tax) |
|---|---|---|
| IV Drip / Local Injection (Adipose-Derived) | Organ, vascular, and tissue regeneration; neural repair and activation; skin & hair quality enhancement | 1V: ¥55,000 3V: ¥165,000 5V: ¥275,000 |
Intranasal Administration (Adipose-Derived) | Cognitive function improvement; may be combined with IV therapy | 1 bottle: ¥49,500 4 bottles: ¥188,100 |
| Treatment | Expected Effects | Price (incl. tax) | IV Drip / Local Injection (Dental Pulp-Derived) | Neural repair and activation; skin & hair quality enhancement | 1V: ¥33,000 3V: ¥99,000 5V: ¥165,000 |
IV Drip / Local Injection (Umbilical Cord-Derived) | Vascular and tissue regeneration; skin & hair quality enhancement | 1V: ¥33,000 3V: ¥99,000 5V: ¥165,000 |
|---|
Note: Mild bruising or discomfort may occur at the injection site.
This therapy is not covered by public insurance.
Treatment effects may vary by individual.
Optional Add-On with Aesthetic Procedures
Stem cell supernatant can be added to the following aesthetic procedures (HART-F, Mesona Plus)as an optional enhancement.| Add-On Option | Price (incl. tax) |
|---|---|
| Adipose-Derived Supernatant | (1V)¥55,000 |
| Umbilical Cord-Derived Supernatant | (1V)¥33,000 |
Combined Trial Packages
| Treatment | PlanDescription | Price (incl. tax) |
|---|---|---|
| HART-F Trial (1 session) + Adipose-Derived Supernatant (1V) | Enhances hair growth by delivering exosome formulation (ASCE+), minoxidil, and supernatant into the scalp's dermal layer | ¥198,000 |
| HART-F Trial (1 session) + Umbilical Cord-Derived Supernatant (1V) | Same as above, with umbilical cord-derived supernatant | ¥176,000 |
| Mesona Plus Full Face Trial (1 session) + Adipose-Derived Supernatant (1V) | Anti-aging skincare with added supernatant for wrinkles, dullness, and sagging | ¥77,000 |
| Mesona Plus Full Face Trial (1 session) + Umbilical Cord-Derived Supernatant (1V) | Same as above, with umbilical cord-derived supernatant | ¥55,000 |
